Model law “On drug provision in the CIS member states”
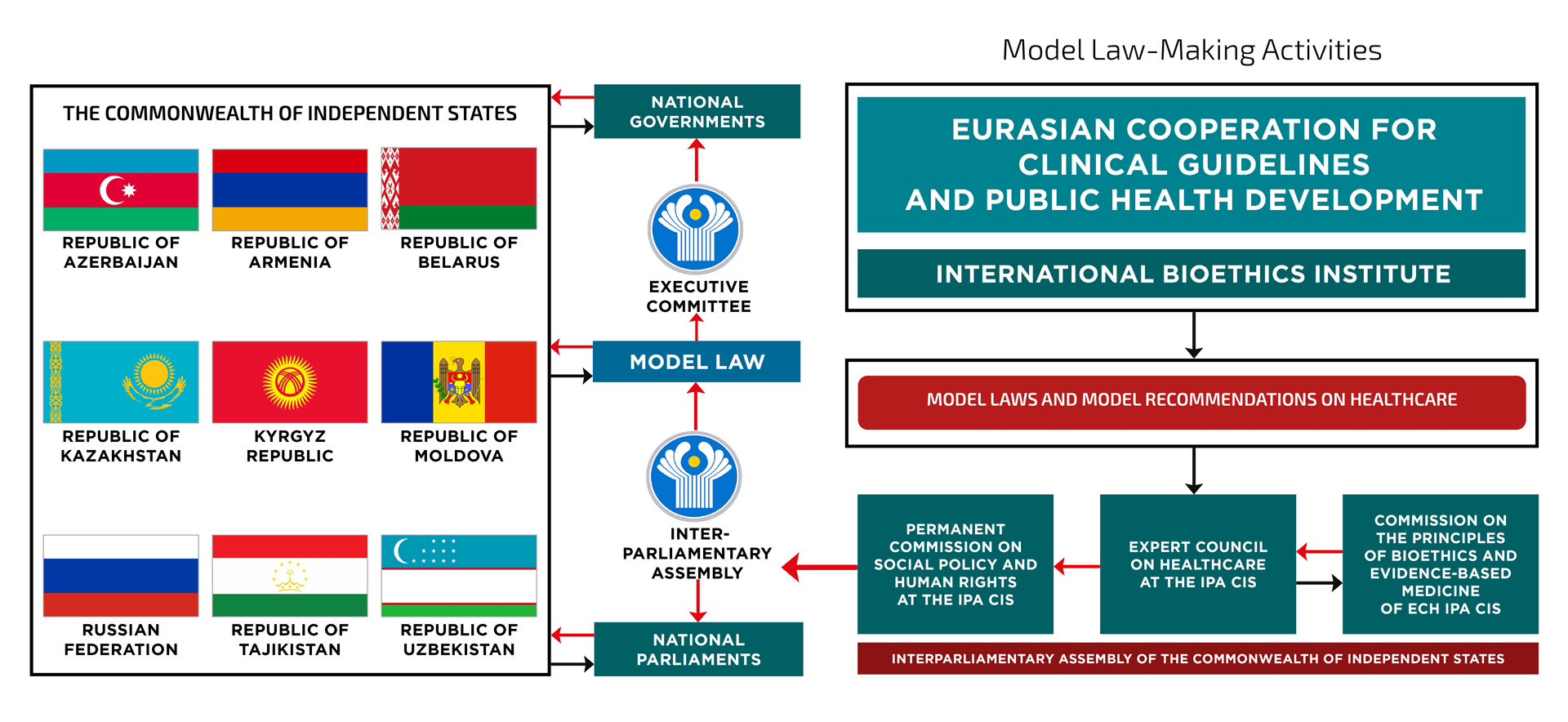
The prospective plan of model lawmaking in the CIS for 2023-2025 envisages the development of a model law “On drug provision in the CIS Member States”. ECHD organizes public expert sessions in the CIS member states to discuss the draft of this model law. ECHD national experts, who are current members of the Expert council on healthcare at the IPA CIS and the Commission on the development of bioethics principles and evidence-based medicine of the ECH at the IPA CIS, actively participate in these expert sessions. For more details on the plan for special expert sessions, please visit the website www.drug-provision.expert
The purpose of adopting the model law “On drug provision in the CIS member states” is to promote legislative harmonization of legal norms on the rights of citizens to drug provision in the CIS member states through the adoption of separate national laws on drug provision or implementation of the developed provisions in general legal acts on medical care, protection of citizens’ health, public health, etc.
The main participants of special expert sessions are:
- Patient communities;
- Proffessional associations;
- Non-profit organizations;
- Pharmaceutical business;
- Insurance companies;
- National parliaments of the CIS member states;
- National ministries of health of the CIS member states;
- National academies of science of the CIS member states;
- Interparliamentary Assembly of Member Nations of the Commonwealth of Independent States;
- CIS Executive Committee;
Main objectives:
- discussion of the draft model law “On drug provision in the CIS member states”;
- identification and consideration of public opinion and expert opinion;
- search for acceptable alternatives to address the most important issues of national importance;
- working out of proposals and recommendations concerning the discussed draft of the model law “On drug provision in the CIS member states”.
Preparation, conducting and establishment of the results of public expert discussions are based on the principles of openness, transparency, voluntariness and independence of experts.

- Creation of expert councils on the provision of drug supply with immunobiologic preparations against the most common infectious and non-infectious diseases in the CIS member states
- Expert analysis of the the role of innovative technologies and drug supply in the prevention, diagnosis, treatment and rehabilitation of diabetes mellitus in the CIS member states
- Analysis of accessibility of drug supply in rehabilitation of disabled people and provision of palliative care
- Analysis of problems of prevention, diagnosis and treatment of viral hepatitis B and C, HIV, rare diseases, as well as diseases characterized by high blood pressure
- Development of definition of the patient as a subject of law and definition of the functionality of the participants of the system of drug provision in the interests of the patient;
- Provision of medicines to citizens of CIS member states included in the national medical register of CIS member state patients for the most common diseases according to ICD-11;
- Ensuring the availability of medicines to citizens of CIS member states in accordance with clinical protocols and recommendations recognized in international evidence-based medicine;
- Development of mechanism of drug provision for CIS citizens in outpatient care -> assessment and management of the quality of medical care;
- Improvement of the mechanism of drug provision for CIS citizens in the hospital link -> evaluation and management of the quality of medical care;
- Development of the method of financial planning of procurement of medicines to meet the needs of patients included in the national medical register of patients;
- Development of the method of determining the optimal price for the state to purchase in the interests of the patient a medicinal product manufactured at a localized pharmaceutical enterprise;
- Development of the method of determining the optimal price for the purchase by the state in the interests of the patient of a medicinal product imported to the CIS member states from other countries;
- Development of the procedure and conditions for conducting clinical trials of medicinal products locally produced in CIS member states;
- Development of the procedure and conditions for conducting clinical trials for imported medicinal products to CIS member states based on the principles of bioethics and evidence-based medicine.
- Definition of the goals and objectives of realization of the state policy in the sphere of drug provision;
- Definition of the rights and obligations of all participants of the health care system in the sphere of drug supply;
- Implementaion of the state registration of medicines for the benefit of patients;
- Implementaion of the mechanisms of mutual settlements, storage, transportation and delivery of medicines to the patient, including the use of pharmacy facilities;
- Implementaion of the procurement mechanism in the interests of the patient and according to the doctor’s prescription;
- Implementaion of the centralization mechanisms of drug supply funds;
- Implementaion of the mechanism for determining an acceptable price when procuring medicines and selling them;
- Monitoring of the effectiveness and safety of medicines and publication of summarized annual results for public information;
- Monitoring and accountability for violations of the rights to medicines provision etc.
Project Progress
Mechanism of model legislation